> Did You Know?
From ore to phosphate
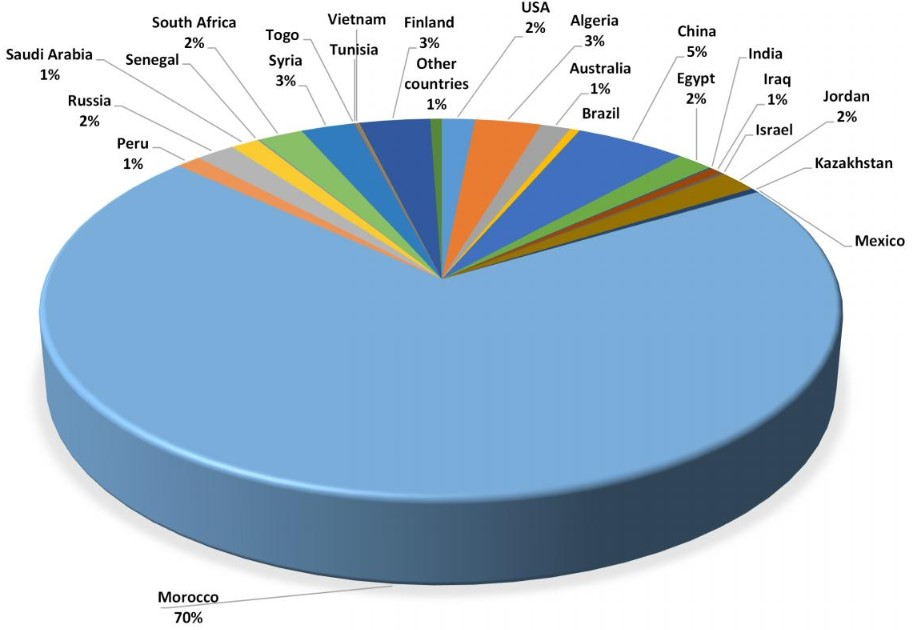
Phosphorus is widespread in the Earth’s crust but is relatively scarce in concentrated forms. The only cost-effective production method to date is the mining of phosphate rock. World phosphate reserves are highly concentrated : only five countries hold about 85 % of known reserves, with 75 % located in Morocco and Western Sahara.
Commercial reserves of rock phosphate (USGS, 2016)
Like with other non-renewable resources, there are huge discrepancies in estimates of the lifetime of phosphate rock reserves, depending on modelling and assumptions on extractable volumes. Depletion scenarios vary from less than 100 years to several hundreds of years. As with the timing of peak oil, the question of peak phosphorus is not settled.
The industrial process for phosphates is based on solubilisation, which includes the production of phosphoric acid (H3PO4). Phosphoric acid is obtained by mixing natural phosphate with sulfuric acid. Sulfuric acid is obtained from sulphur, but also from the fatal acid of the metal industry (copper / zinc). Essential phosphoric acid is, thus, the basis of most phosphate products for use in agriculture (about 80-85%), but also in animal feed, food, industry, detergent industry, etc. MAP/DAP (Mono/Diammonium Phosphates) fertilisers are manufactured by adding ammonia.
