> Animals
Bone demineralisation
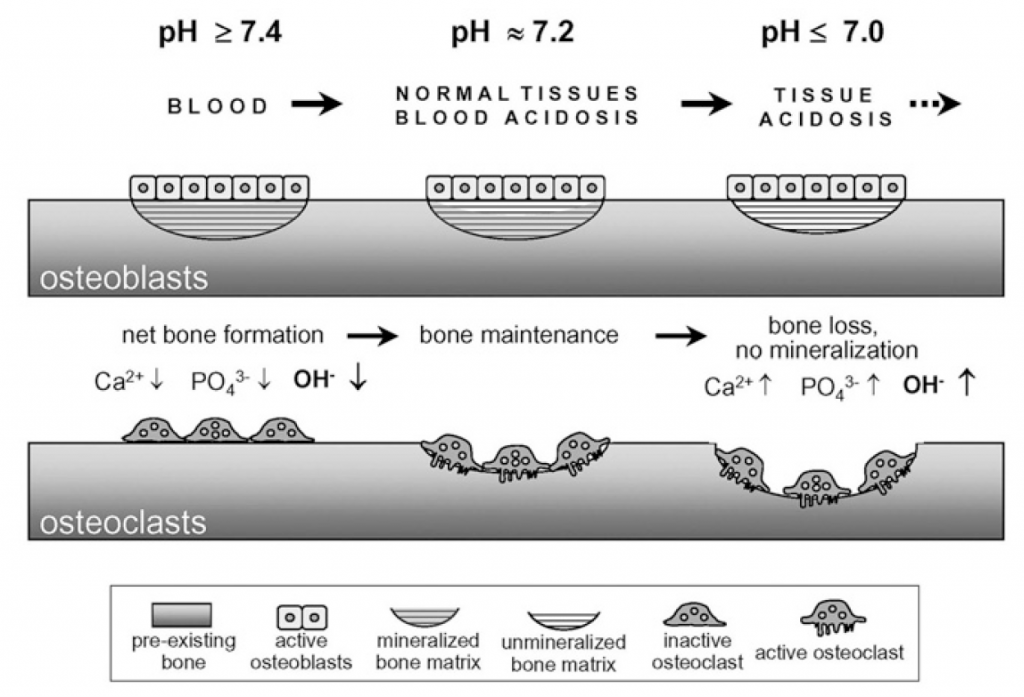
Metabolic machinery produces acid which needs to be buffered and eliminated : pH in the blood and extracellular fluid must remain within narrow limits. With a massive reserve of alkaline mineral (hydroxyapatite), the skeleton of land vertebrates acts as proton buffer. Calcium efflux from bone is induced by metabolic acidosis. Excess H+, for example as a result of the metabolism of sulfur-, nitrogen-, and phosphorus-containing molecules, will decrease pH. Chronic metabolic acidosis decreases cortical bone mass (a prime determinant of bone fragility) and impairs bone microarchitecture.
Effects of extracellular pH on bone formation and resorption
Osteoclasts: cells that break down bone tissue for the maintenance, repair, and remodelling of bones
Electrolytes such as Na, K, Cl, S… are minerals capable of dissolving and dissociating into positively and negatively charged ions. They contribute to homeostatic regulation of acid-base balance. The risk of metabolic acidosis is increased with the acidification of the diet, with the reduction of protein sources rich in K+, with sulfur-rich ingredients, under heat stress conditions, and by diarrhea (loss of HCO3–). Dietary Electrolytic Balance (dEB) in feed formulation is calculated by the difference between Na+ and K+, which have indirect alkalogenic effects on body fluids, and Cl− ion which has an indirect acidogenic effect. The role of sulfur in dietary anion-cation balance is well accepted in dairy but seems underestimated in monogastric animals. Too low dEB, favoured by excess Cl− or S–, induce skeletal problems on legs and articulations. Feeding an alkalinogenic diet may conserve mineral reserves and help reduce lameness in food producing, reproductive and companion animals.